Alzheimer’s Disease
What Is Alzheimer’s Disease?
Alzheimer’s disease is a neurodegenerative disease that primarily affects individuals over 65 years of age. It is characterized by a progressive loss of memory due to the dysfunction and loss of brain cells (neurons) in brain areas important for cognition.
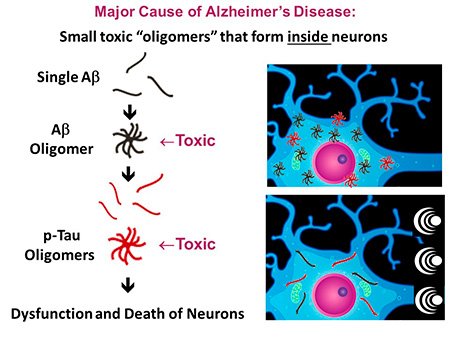
While the exact cause of Alzheimer’s is unclear, many Alzheimer’s researchers believe the root cause is an aggregation of two abnormal proteins (Aβ and p-tau) that are present inside neurons.
Initially, single Aβ proteins self-aggregate to form Aβ oligomers, which then stimulate the phosphorylation of the second protein into p-tau and aggregation of single p-tau proteins into p-tau aggregates (see figure on the right). Multiple toxic processes are induced by these Aβ and p-tau oligomers that result in the dysfunction or death of neurons. The presence of both these oligomers inside neurons is an early pathogenic event, occurring before overt signs of Alzheimer’s memory loss.
To our knowledge, there is no known Alzheimer’s therapeutic that has been shown to break up Aβ and p-tau oligomers inside neurons – which is where they appear to cause the dysfunction and death of neurons.
NeuroEM has shown that Transcranial Electromagnetic Treatment leveraging Radio Frequencies (TEMT-RF) breaks up both Aβ and p-tau oligomers inside and outside neurons. Although this is probably TEMT-RF’s primary mode of action against Alzheimer’s, there are several other beneficial actions of TEMT-RF that NeuroEM has identified, including an increase in neuronal energy production (mitochondrial enhancement). TEMT-RF also rebalances the immune system in both the blood and brain to reduce inflammation.
-
No cognitive impairment, but loss/dysfunction of neurons starts to occur. This stage begins 10 or more years before any noticeable memory loss.
-
Modest impairment of short-term (immediate) memory as neuronal loss/dysfunction continues.
-
Substantial short-term and long-term memory impairment, with eventual total loss of intellect – all this over the course of 2 to 20 years.
Three Stages of Alzheimer’s Disease:
Alzheimer’s Prevalence, Cost & Demographics
With half a million Americans diagnosed every year,
Alzheimer’s is among the costliest conditions to society.
Current Alzheimer’s Drugs
There is currently no effective preventative or treatment for Alzheimer’s.
At this time, FDA-approved drugs only temporarily slow the rate of cognitive decline, if at all. These drugs only mask the disease’s symptoms and are not disease-modifying. To date, all clinical trials involving drug treatment in Alzheimer’s patients have universally failed to provide long-term benefits.
For over 20 years, four drugs have been FDA-approved to treat Alzheimer’s disease (three acetylcholinesterase inhibitors and memantine). These drugs only temporarily slow the rate of cognitive decline, if at all, they mask the disease’s symptoms, and are not disease-modifying.
Within the past several years, the FDA has fast-track approved two monoclonal antibodies (MCAs) against Aβ. The MCAs attack various aggregated forms of Aβ – but only outside of neurons. These MCAs, aducanumab and lecanumab, have been reported to slow the cognitive decline of Alzheimer’s patients by around 30%, but when alternative statistical analysis of the data is done, the slowing is only 8-10%, which is of no clinical significance.
Moreover, these MCAs can have significant side effects, including brain microhemorrhages and brain shrinkage. To date, all clinical trials involving drug treatment in Alzheimer’s patients have universally failed to reverse their cognitive impairment or to stop their cognitive decline long-term.
Not only do Alzheimer’s drugs generally fail to effectively get past the blood-brain barrier, but more importantly they have extreme difficulty getting inside the brain’s neurons – which is where oligomeric Aβ and oligomeric p-tau (the probable causes of Alzheimer’s memory impairment) reside. Even if they did get into neurons, current Alzheimer’s drugs generally do not target these two oligomeric proteins.
NeuroEM’s electromagnetic technology, which directly and uniquely attacks both Aβ and p-tau oligomers inside neurons, may represent an effective treatment in the near future that stops and reverses Alzheimer’s cognitive decline.
Other Neuromodulation Approaches
In addition to TEMT-RF, several other neuromodulatory (non-drug) approaches are currently being clinically investigated as potential treatments for Alzheimer’s.
Transcranial Magnetic Treatment (tMS) involves clinical visits to administer magnetic waves to the brain through several large magnets placed on the head during each treatment.
Deep Brain Stimulation (DBS) requires invasive surgical implantation of several electrodes deep into the brain; the electrodes are then connected to a subcutaneously placed battery/activation unit.
Both tMS and DBS provide stimulation effects on neuronal activity but have thus far not been shown to affect the memory decline of Alzheimer’s patients in Phase II/III clinical trials or to affect the Alzheimer’s process.
Transcranial Ultrasound (tUS) applies ultrasound to the head to open up the blood/brain barrier, which apparently allows some unknown agent to enter the brain from the blood to activate certain brain cells.
tUS requires clinical MRI monitoring before and after treatment, with potentially toxic gadolinium being infused prior to scanning. The first clinical trial with tUS in Alzheimer’s patients is currently underway.
Photomodulation involves treatment with flickering LED or infrared light at 40 Hz via glasses (Cognito Therapeutics) or transcranial/intranasal treatment (Vielight).
How photomodulation might benefit Alzheimer’s patients is currently unknown, although gamma wave entrainment has been proposed. Also, particularly for Vielight’s device, studies have shown that light can only penetrate the bony cranium of humans at very high power levels.